Academics
Effect of a Probiotic on Gut Health and Fecal Microbiome Changes
Effect of a Probiotic on Gut Health and Fecal Microbiome Changes
LU IRB#: IRB-25-41
PI: Chad Kerksick, PhD
Description of Study
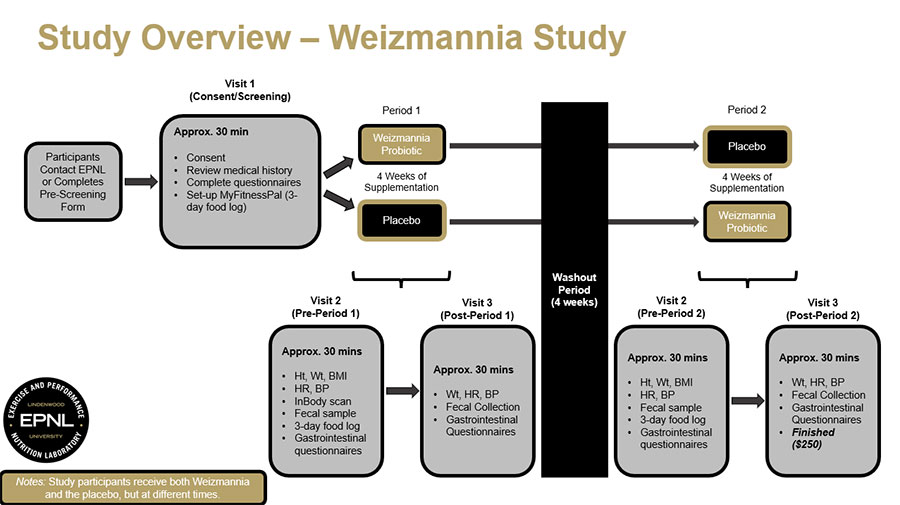
Participate in this study to help us learn about how Weizmannia (Bacillus) coagulans, a probiotic, can impact your gut health. A probiotic is a live microorganism that, when consumed in adequate amounts, provides health benefits by promoting the growth of beneficial bacteria in the gut. Learn more about this study.
The purpose of this study is to examine how supplementation with Weizmannia (Bacillus) coagulans JBI-YZ6.3 affects gut health in healthy, physically active men and women.
How Do I Get Involved?
- Review the Study Overview
- Review the Information Brochure
- Complete the Initial Screening Form
Points of Contact
James Tice
[email protected]
Research Sample Needed
- 30 healthy, physically active males and females who occasionally experience mild to severe bloating
Criteria
- Ages of 18 – 50 years
- Average daily bloating score >5 on question 3 of the Modified daily Abdominal, Gas, and Bloating Questionnaire and/or bloating for >5 days during the past 14 days
- Body mass index (BMI) 18.5 – 29.9 kg/m2 (Inclusive) (Individuals >29.9 kg/m2, but <25% fat for men and <30% fat for women will be accepted into the study. The cohort average of body mass index will not exceed 29.9 kg/m2)
- Weight stable for the past three months (defined as less than a 5% variation in body mass over this time)
- Determined to be healthy through completion of a health history questionnaire
- Subject agrees to maintain their existing dietary patterns throughout the study period and to report to study investigators any changes particularly as they relate to probiotic-containing or fermented foods.
- Subject agrees to refrain from alcohol, caffeine, and strenuous exercise for 24 hours prior to each test day.
- Minimum baseline physical activity level (defined as at least 30 minutes of moderate intensity exercise at least 4 days per week for the past 3 months)
- Subject is willing and able to comply with the study protocol.
- Study participant is not currently enrolled in another clinical trial that involves the administration of some investigative agent.
- Subject has given voluntary, written, informed consent to participate in the study.
- Positive medical history and/or is currently being treated for some form of heart disease, cardiovascular disease
- Currently being treated for kidney disease, renal failure, or has dialysis performed on regular intervals
- Has liver disease or some form of clinically diagnosed hepatic impairment
- Diagnosed with having Type I or Type II diabetes (determined as fasting blood glucose > 126 mg/dL)
- Diagnosed with or is being treated for some form of thyroid disease
- Diagnosed with major affective disorder or other psychiatric disorder that required hospitalization in the prior year
- Diagnosed with some form of immune disorder (i.e., HIV/AIDS)
- History of cancer (except localized skin cancer without metastases or in situ cervical cancer within 5 years prior to screening visit).
- Participant has an abnormality or obstruction of the gastrointestinal tract precluding swallowing (e.g., dysphagia) and digestion (e.g., known intestinal malabsorption, celiac disease, inflammatory bowel disease, chronic pancreatitis, steatorrhea)
- Participant has been treated for a gastrointestinal related disorder, complication, or disorder within the past 30 days
- Positive medical history for any neurological condition or neurological disease
- Diagnosed with or being treated for any endocrinological disorder or currently used any form of hormone replacement (prescribed/doctor ordered or not)
- Women with a history of hormone-related conditions such as endometriosis, fibroids, polycystic ovary syndrome
- Currently prescribed for the first time statin drugs (i.e., Lipitor, Livalo, Crestor, Zocor, etc.) within the past 6 months or has had their dosage or medication changed within the past 6 months
- Currently prescribed for the first time hypertension medication (i.e., Beta-blockers, ACE Inhibitors, Alpha blockers, Vasodilators, etc.) within the past 6 months or has had their dosage or medication changed within the past 6 months
- Current antibiotic use or other prescription or over-the-counter medications that may impact study outcomes
- Have a known sensitivity or allergy to any of the study products
- Blood donation in past 60 days
- Current smoker (average of > 1 pack per week within the past 3 months) or has quit within the past six months. This includes all forms of nicotine
- They plan major changes in lifestyle (i.e., diet, dieting, exercise level, travel, etc.) during the study
- Competitive athletes will be excluded
- History of alcohol or substance abuse in the 12 months prior to screening
- Current use of anabolic steroids (medically prescribed or otherwise)
- Receipt or use of an investigational product in another research study within 30 days of beginning the study protocol
- Report taking a probiotic or other dietary supplement know to impact digestion or gut function in the past 30 days
- Recent history (<3 months) of exercise training or weight loss (> 5%)
- Currently following a ketogenic or low carbohydrate diet within the past 30 days.
- Women who are pregnant, planning to become pregnant, or lactating currently or within the past six months
- Any condition or abnormality that, in the opinion of the investigator, would compromise the safety of the participant or the quality of the study data
Are You Eligible?
Information provided here reflects current IRB approval for this research. However, this information may be subject to change and updated accordingly.
Dr. Chad Kerksick
Director, Exercise and Performance Nutrition Laboratory (EPNL)