Academics
Long-Term Creatine Supplementation Study in Adolescent Athletes
Long-Term Creatine Supplementation Study in Adolescent Athletes
LU IRB#: IRB-23-87
PI: Chad Kerksick, PhD
Description of Study
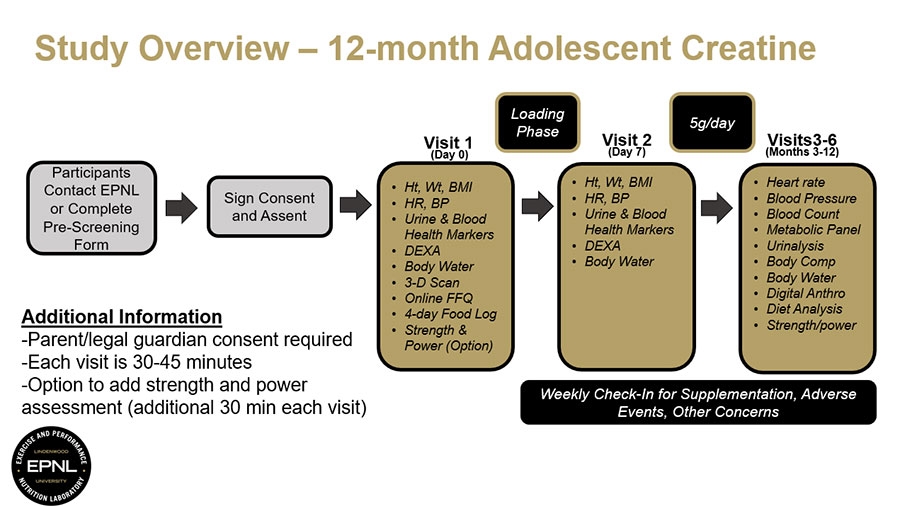
Participate in this study to help us learn about how long-term creatine monohydrate supplementation may affect health, body composition, and strength and power in high school athletes. Creatine is a naturally occurring compound found in foods and produced by the body, and it is commonly used to support strength and high-intensity exercise performance. Learn more about this study.
The purpose of this study is to evaluate the effects of long-term creatine monohydrate supplementation on various indicators of health and safety, body composition, and strength and power after 12 months of supplementation in high school athletes.
How Do I Get Involved?
- Review the Study Overview
- Review the Information Brochure
- Complete the Schedule Online Info Visit
Points of Contact
Sophie O’Cain
[email protected]
Research Sample Needed
- 100 high-school-aged athletes
Criteria
- Each participant must be cleared medically by their school or a medical professional for sport participation
- Approximately 100 male and female high school athletes of all racial backgrounds, between the ages of 15 – 17 years of age will be recruited for this study
- Participants will be required to report their typical nutritional intake including any other dietary supplements as well as exercise training protocols throughout the study protocol
- All participants will abstain from ingesting all foods, fluids, supplements (including caffeine), and medications for at least 8 hours prior to testing
- All participants who are already taking creatine will be required to stop taking creatine for 30 days prior to enrolling in the study
- All participants will be required to refrain from unaccustomed exercise for at least 24 hours prior to each testing session and have not completed any exercise in the previous 12 hours
- Any combination of information determined as part of the review of information through completion of the proposed data safety monitoring plan (DSMP) that results in a recommendation for any participant to be excluded or removed from participation
- Any individual who is not medically cleared by their school or medical professional to participate in this study will be excluded from this study.
- Athletes who are currently recovering or rehabilitating a musculoskeletal injury (e.g., bone fracture, muscle tear or rupture, tendon strain or rupture, ligament sprain or rupture). In these instances, the potential participant may begin the study protocol after being medically cleared to begin participating in sporting activities.
- Any participant currently taking thyroid, anti-hyperlipidemic, hypoglycemic, anti-hypertensive, anti-inflammatory, or androgenic medications. Other medications could be deemed exclusionary based upon clinical review by study physicians.
- Any participant who has taken any nutritional supplements or ergogenic aids (i.e. creatine, beta-alanine, HMB, DHEA, thermogenic, etc.) other than a daily multivitamin and protein powder within the previous 4 weeks
- Any athlete who is younger than 15 years of age or older than 17 years of age will be excluded.
- Participants who are lactating, pregnant or planning to become pregnant
Are You Eligible?
Information provided here reflects current IRB approval for this research. However, this information may be subject to change and updated accordingly.
Dr. Chad Kerksick
Director, Exercise and Performance Nutrition Laboratory (EPNL)